Klinicheskiye tests of the latest – long and labor-intensive process. To create new means, years of researches are required. But even quite successful passing of the first stages of test of new preparations cannot insure from accident.
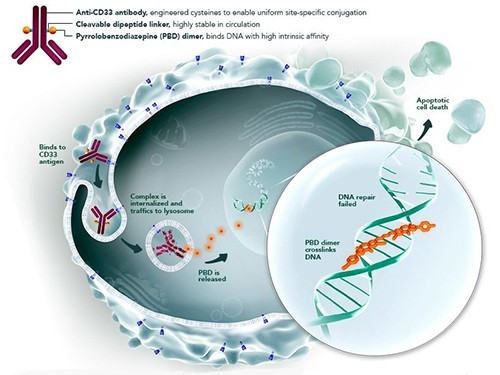
In December of last year the administration on behind and drugs of United States of America (FDA) imposed a ban on clinical trials of a perspective experimental preparation from a sharp myeloid leukosis (AML) of vadastuksimaba talirina (vadastuximab talirine). According to data of the Seattle Genetics company owning the rights for these , during clinical trials gepatotoksichnost signs were found in six patients with sharp the miyeloleykozy, four participants of clinical tests died.
of Vadastuksimab talirin (SGN-CD33A) — the antibody loaded with , specific to CD33 protein. This protein expressiruyetsya on the majority of cells of a sharp amiloidny leukosis. The preparation kills cancer cells, leaving healthy fabrics intact. Researches showed that the substance really causes remission in patients.
In March the ban on further tests of a preparation was removed by, and researches on group of 300 participants were continued. However the other day the Seattle Genetics Inc declared that completely stops research of a perspective preparation on a closing stage of tests because of higher mortality at the patients accepting a preparation, in comparison with the control group accepting placebo. Representatives of Seattle Genetics refused to open data on amount of death, but thus declared that all cases of death were not connected with a preparation gepatotoksichnost.

For development vadastuksimaba talirina which was considered earlier very perspective, were spent huge amounts of money and time. After news of a full stop of tests of the share of the company sharply fell in price, and analysts predicted considerable reduction of revenue of Seattle Genetics up to 2021.
Knows that the majority (about 90%) latest which reach a stage of clinical tests, these tests do not take place. Even at achievement of the III stage — and drugs which, obviously, safely passed selection at the previous stages reach it only — the percent of failure makes 40%. Development of medicine — is a unique combination of the highest legislative loading and very high percent of failures so there is a temptation to tell what exactly the rigid legislation serves as the reason of these failures. But it not so. the Reason — in improbable complexity of process.
C 1979 for 2005 the cost of development grew from about 100 million dollars to 0,8-1,2 billion dollars.
For the last decade procedures of clinical trials considerably became complicated. From 1999 to 2005 the number of analyses, inspections and pr within one clinical trial grew with 96 to 158 (65%). Thus level of a set of patients (number of the patients, satisfied to more and more rigid selection criteria and included in research) fell with 75 to 59%, and the number of the patients who have finished research — decreased with 69 to 48% (30 %). Duration of clinical trials grew from 460 to 680 days.
Why it is so difficult?
Development of medicine at first takes place preclinical researches (on biological models, "in Petri's cup"), then test for laboratory animals. Only at safe passing of all stages (from mice to primacies) further researches get approval of ethical committee and the positive decision of authorized body of health care of that country where it is planned to conduct research. Only after that it is possible to pass to clinical tests which are subdivided into three major stages.
- I stage. The preparation passes the first test on the person which as a whole is directed on an assessment of shipping by a human body, in it takes part from several tens to about 100 people — healthy volunteers. If a preparation highly toxic (for oncology treatment, for example), patients take part in research with the corresponding disease.
- II stage. He assumes participation in tests already several hundred patients having a certain disease for which treatment the studied preparation is intended. The main objective of the II stage of clinical tests is the choice of the most suitable therapeutic dose of a studied preparation.
- III stage. Preregistration research with participation already several thousand patients of different age, and, as a rule, from the different countries, for obtaining authentic statistical data which will be able to confirm and efficiency .
the First stage of clinical trials, certainly, is the most dangerous. For this reason it passes only in specialized institutions, specially for this purpose equipped. In them participants of experiment at any stage can receive the fastest and qualified help if something goes not so. Most often such researches conduct to United States of America, Netherlands and Canada. In many countries such experiments are forbidden by the law. So, to Russian Federation it is imposed will lock on research of foreign preparations with participation of healthy volunteers. However, this ban does not extend on patients.
To Russian Federation the market of clinical trials cardinally changed in 2013: from mainly innovative it turned into the dzhenerikovy. The patent collapse on a world pharmaceutical market became the reason of this change and reaction of foreign producers to the law "About the Address of " according to which for registration foreign to Russian Federation it is necessary to present results of researches with participation of the Russian clinical centers.
B 2015 a number of federal laws, which made essential changes into the Federal law of 12.04.2010 No. 61-FZ "About the address of " came into force. In particular, the conceptual framework of the law (the special attention was paid on reference and orfanny ) was processed, new types of are added, procedure of registration of preparations, an order of state regulation of the prices of vital and major is changed (ZhNVLP), parameters of interchangeability and many other things are established. The adopted amendments had to simplify process of development and tests of new preparations, however in practice procedure became even more difficult.
However such mechanism is absolutely necessary: the majority of the preparations which have reached clinical tests, are ineffective. If to ask system (and, therefore, to reduce the price), the set not until the end of the checked preparations will get to a distribution network that can cause unpredictable and often irreversible consequences (as it happened already once to a preparation under the name "talidomid"). For this reason expensive, long, extensive and multi-stage clinical tests are absolutely necessary for any that, of course, considerably increases their final cost in case of successful passing of all stages. It leads the following problem: if for widespread diseases such clinical tests are economically justified, development medicine for rare, orfanny diseases has very low chances. And it still remains an unsoluble ethic and economic problem.